Antibiotic resistance: Breaking the cycle

Antibiotic resistance could become one of the greatest problems of our generation, given the increasing rise in bacterial strains that are less sensitive to existing treatments. While abuse of antibiotics in humans is probably the major contributor, policymakers have turned the spotlight on agricultural use as a way to control the problem.
Each year, the ECDC and EFSA produce a summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food. Such data reveals some interesting insights and highlights the occurrence of resistance to commonly used antimicrobials in veterinary medicine in carcasses of food-producing animals.
The report for 2018/2019 indicates that among Salmonella spp. recovered from broiler carcasses, resistance to the fluoroquinolone antimicrobial agents ciprofloxacin and nalidixic acid was reported at high to extremely high levels in many member states in the EU, with overall resistance at 51.4% and 48.8%, respectively.
Information concerning the antimicrobials ampicillin, sulfamethoxazole and tetracycline is equally noteworthy. As high-priority, ‘critically important antimicrobials’ (CIAs) and ‘highly important antimicrobials,’ it is of concern that overall resistance to them remained very high among Salmonella spp. recovered from poultry carcasses.
Likewise, in relation to most recent information on resistance to fluoroquinolones, which are the highest priority CIAs in human medicine, is of even greater concern given that their use in food-producing animals is subject to prudent use initiatives. The report for 2018/2019 indicates that among Salmonella spp. recovered from broiler carcasses, resistance to the fluoroquinolone antimicrobial agents ciprofloxacin and nalidixic acid was reported at high to extremely high levels in many member states in the EU, with overall resistance at 51.4% and 48.8%, respectively.
Other trends indicate that the relative resistance rates for S. typhimurium remain static, but this should still be a concern due to the relatively high rates noted. The majority of antibiotic resistance can be noted among broilers, and this may, in part, be due to the lower level of antibiotic use in the layer industry relative to the broiler market.
The data further indicates a high level of antibiotic resistance amongst isolates of Campylobacter, with high to extremely high levels of resistance to antimicrobials such as ciprofloxacin, nalidixic acid and tetracycline being noted in meat samples of broilers. Of particular interest is the high rate of resistance to CIA antibiotics, such as fluoroquinolones in C. jejuni and C. coli in broiler meat.
What are the alternatives?
While the debate rages over what is driving the seemingly inexorable rise of antibiotic-resistant microbes, alternatives to antibiotics and products capable of reducing the risk of antibiotic resistance transfer through the food chain are clearly required. Globally, it is recognised that there is no so-called “silver bullet” to replace antibiotic use in animal production and producers will almost certainly have to improve hygiene and husbandry to address the issue.
Products that will assist the move to antibiotic-free production status include many that are designed to regulate and support the gut environment and its microflora, such as:
- Coccidial vaccines
- Probiotics
- Competitive exclusion products
- Feed enzymes
- Functional nutrients, such as nucleotides
- Organic acids and feed hygiene products
- Organic minerals
- Plant-based products, such as herbs, spices and essential oils
- Yeast cell wall derivatives, such as mannan-oligosaccharides (MOS) and mannose-rich fractions (MRF)
Of the functional ingredients currently in use for microbial control, mannan-oligosaccharides (MOS) are widely used in animal nutrition and have been shown to improve animal performance in a manner similar to antibiotic-like growth promoters. Having been commercially available since their launch in the early 1990s, a substantial body of scientific papers and practical examples of MOS efficacy has been generated. Since 1999, the use of MOS in animal feed has become more prominent, mainly due to the European ban on prophylactic antibiotic growth promoters in animal feed. Given their ability to bind and limit the colonisation of gut pathogens, MOS has proven to be an effective solution for pathogen control, as well as supporting overall health and performance.
While the early use of yeast mannan products was linked with control of pathogens such as Salmonella and E. coli, further refinements of yeast MOS have led to the isolation of a mannose-rich fraction (MRF) with enhanced benefits for intestinal health.
Recent studies on MRF have focused on the impact that these more refined carbohydrate fractions have on the overall bacterial community of the poultry gut. Such work has shown that MRF supplementation of poultry diets can significantly enhance the diversity of the intestinal microflora (the so-called microbiome), and in doing so, decrease the prevalence of microbial pathogens, such as Campylobacter, which are of interest to human health.
In terms of developing strategies to reduce or limit the use of antibiotics, perhaps an elegant solution is to find ways to make the therapeutics more effective. MRF supplementation of the diets of broilers has been associated with a decrease in selected antibiotic resistance gene copy number. This is potentially linked to the ability of MRF to reduce plasmid transfer between microbes (Figure 1), and in doing so, prevent the spread of antimicrobial resistance.
Figure 1 – MRF reduces spread of antimicrobial resistance.
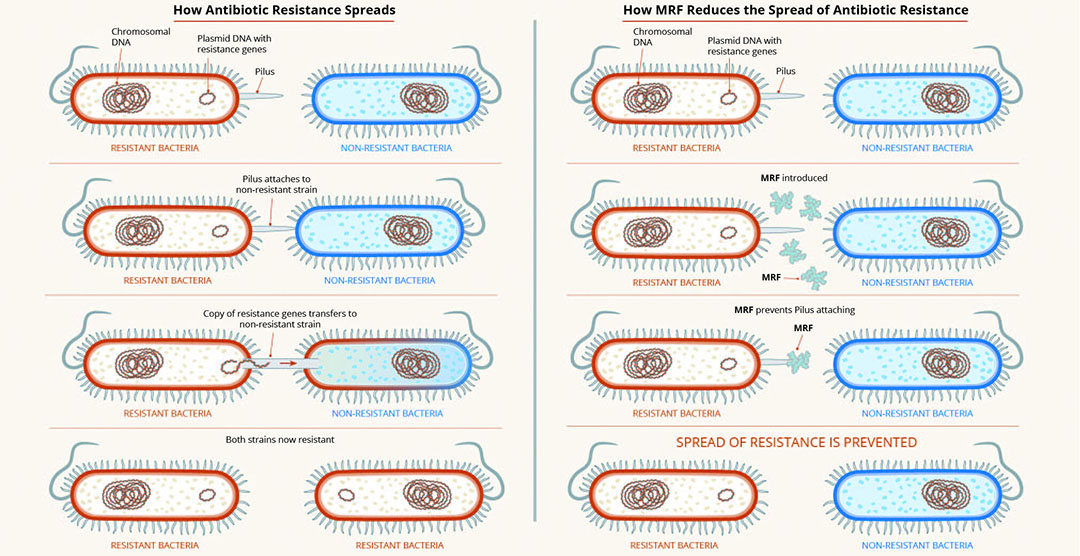
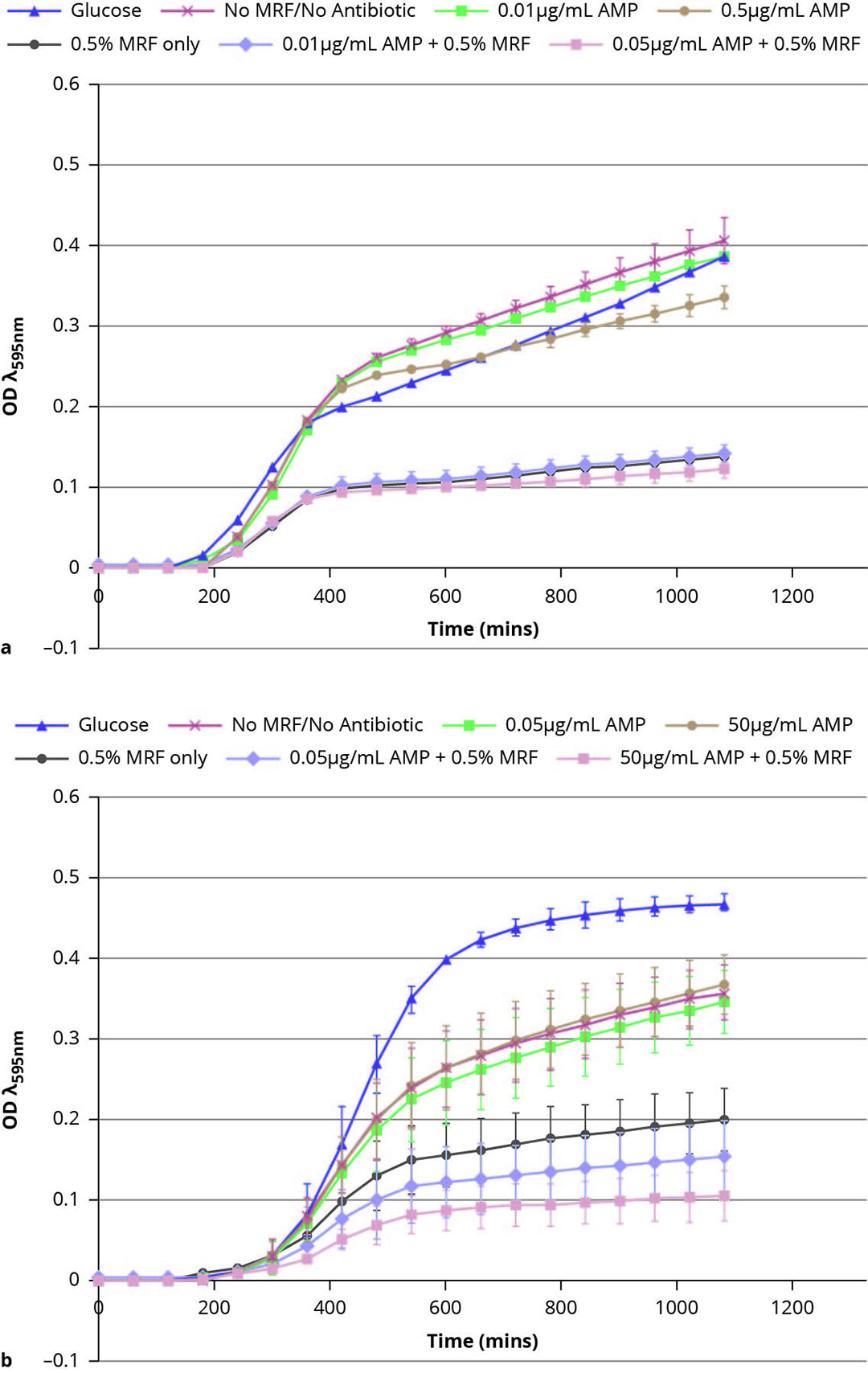
As part of ongoing efforts to support restriction and non-therapeutic use of antimicrobials in the poultry and pig industries, recent research at Alltech has focused on the mechanisms surrounding antimicrobial resistance and its impacts on antimicrobial efficacy toward common pathogens, such as resistant E. coli. Newly published research from the team at the Alltech European Bioscience Centre in Dunboyne, Ireland, has shown that MRF can influence bacterial metabolism, and in doing so, influence the sensitivity of resistant bacteria to antibiotics. This new research demonstrates that when resistant E. coli is grown in the presence of MRF, its growth and metabolism are altered, resulting in antimicrobial-resistant strains becoming increasingly sensitive to antibiotic treatment (Figure 2).
The use of MRF in this instance has been shown to enhance the sensitivity of bacteria to the effects of antibiotics, and in doing so, potentially reduce the minimum inhibitory concentration (MIC) required.
Figure 2 – MRF modulates growth of susceptible and resistant E. coli. Growth curves of (a) antibiotic-susceptible E. coli. and (b) antibiotic-resistant E. coli supplemented and not supplemented with MRF (0.5%) in the presence and absence of ampicillin (AMP).
The future of antibiotics in animal production
From a production standpoint, it is essential that any moves toward antibiotic-free production systems improve overall feed quality, as animals that are fed quality feeds are less susceptible to enteric problems. Ultimately, this move from least-cost feed formulation and reliance on antibiotics will be toward high-quality feeds containing functional ingredients.
Programmes that employ a holistic approach to health management have proven to be extremely effective. By utilising a combination of strategies, producers can rehabilitate and accelerate the evolution of the intestinal microbiota. The success of these “Seed, Feed and Weed” programs is reliant on first seeding the gut with favourable microflora using a probiotic; feeding the favourable microbes through acids or enzymes and, finally, weeding out pathogens by using MRF products.
Conclusions
Concerns about antibiotic resistance among scientists, regulators and consumers have driven the EU ban on AGPs and been a catalyst for change in the US.
This has heralded a global move to reduce antibiotic usage, and further ongoing changes in animal production systems are likely to be substantial.
Ultimately, what is required are innovative replacement products and alternative strategies. The use of functional feed components, such as MRF, represents one such innovative approach to break the cycle of resistance.
Join 13,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the dairy sector, two times a week.






